| inbluevt | Date: Thursday, 2013/07/11, 11:46 AM | Message # 1 | DMCA |
|
|
Private
Group: Blocked
Messages: 1024
|
The chemistry of the ocean is changing. Most climate change discussion focuses on the warmth of the air, but around one-quarter of the carbon dioxide we release into the atmosphere dissolves into the ocean. Dissolved carbon dioxide makes seawater more acidic—a process called
ocean acidification—and its effects have already been observed: the
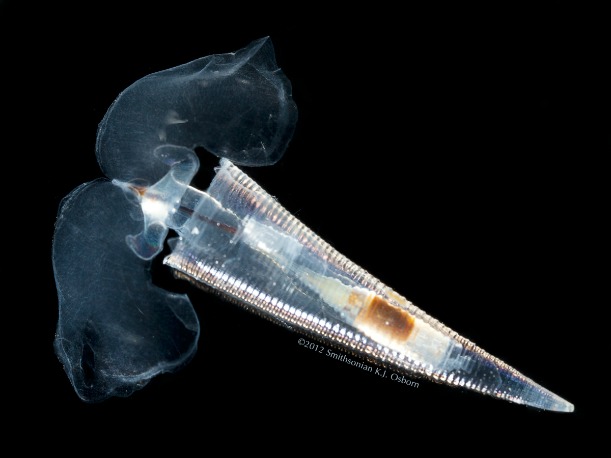
shells of sea butterflies, also known as pteropods, have begun dissolving in the Antarctic.
Tiny sea butterflies are related to snails, but use their muscular foot to swim in the water instead of creep along a surface. Many species have thin, hard shells made of calcium carbonate that are especially sensitive to changes in the ocean’s acidity. Their sensitivity and cosmopolitan nature make them an alluring study group for scientists who want to better understand how acidification will affect ocean organisms. But some pteropod species are proving to do just fine in more acidic water, while others have shells that dissolve quickly. So why do some species perish while others thrive?
More
Message edited by inbluevt - Thursday, 2013/07/11, 11:47 AM |
|
|
| |

 Main
Main  Forum
Forum Registration
Registration Login
Login